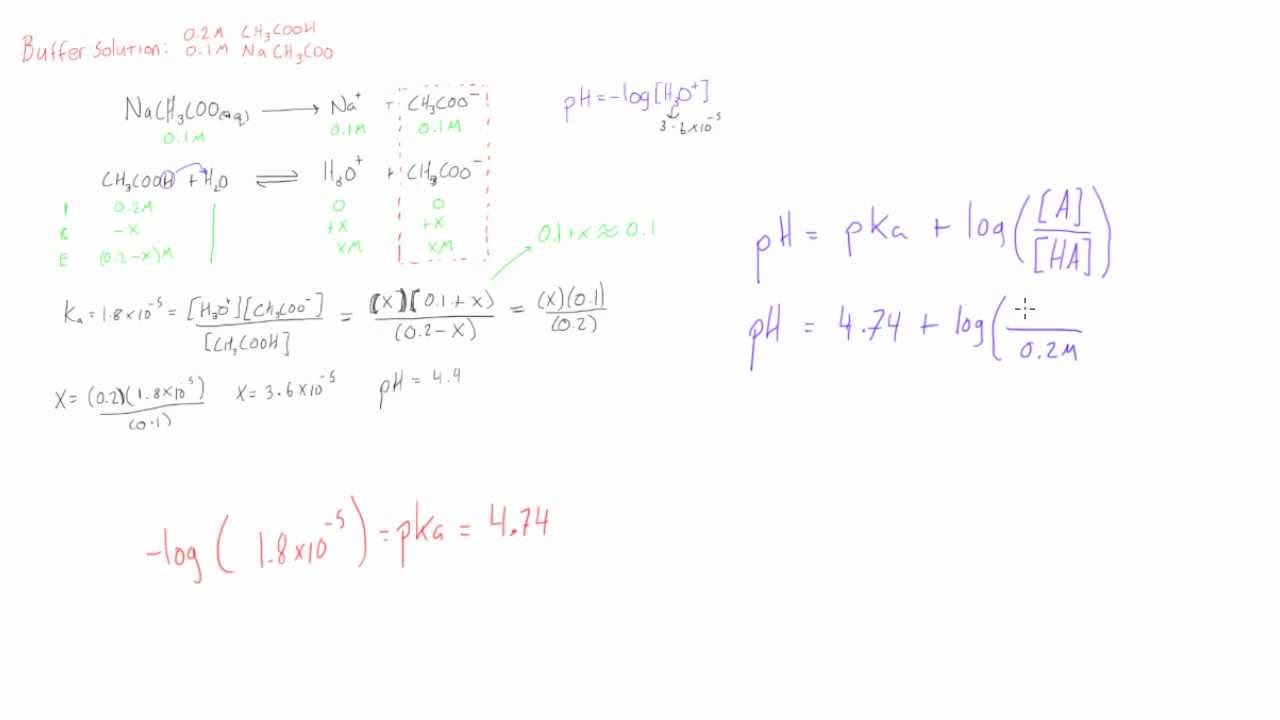Ph Of Buffer Solution Equation
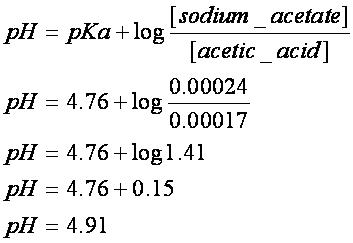
In chemistry the hendersonhasselbalch equation describes the derivation of ph as a measure of acidity using pk a the negative log of the acid dissociation.
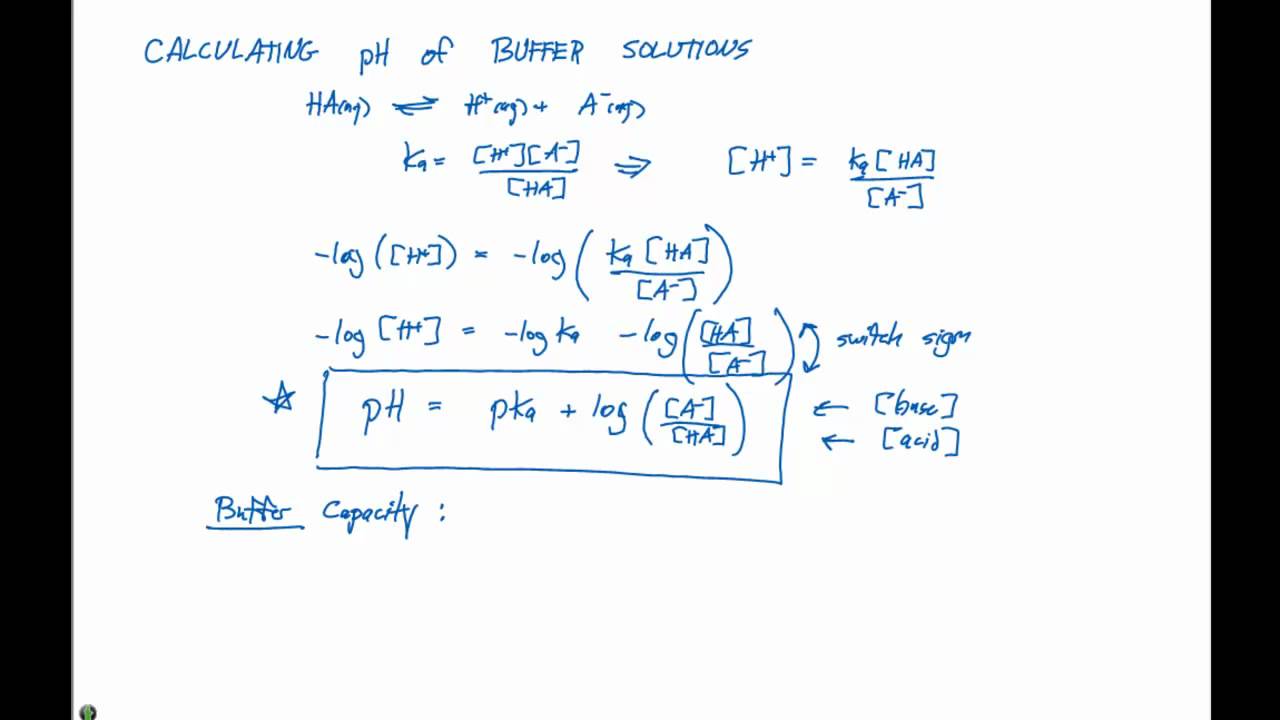
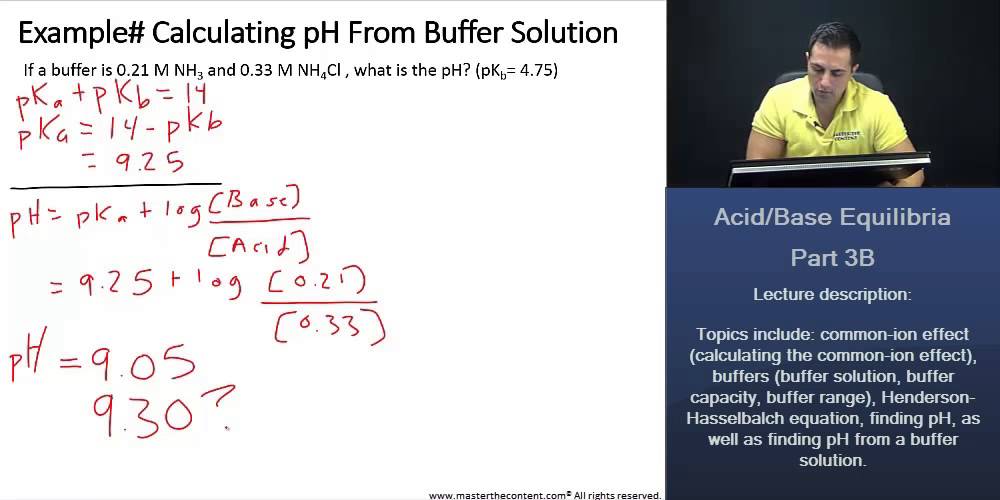
Ph of buffer solution equation. Ph calculation lectures ph of a buffer the henderson hasselbalch equation. Solutions able to retain a constant ph regardless of small amounts of acids or bases. This chemistry video tutorial explains how to calculate the ph of a buffer solution using the henderson hasselbalch equation. It explains the concept.
Example of calculating the ph of a buffer solution using the henderson hasselbalch equation including the ph of the buffer solution after adding some naoh. How do buffer solutions work. A buffer solution has to contain. Where you have done calculations using this equation.
Suppose you wanted a buffer with a ph. You can calculate the ph of a buffer solution or the concentration of the acid and base using the henderson hasselbalch equation. Heres a look at the henderson. A buffer solution more precisely ph buffer or hydrogen ion buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa.
A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base.