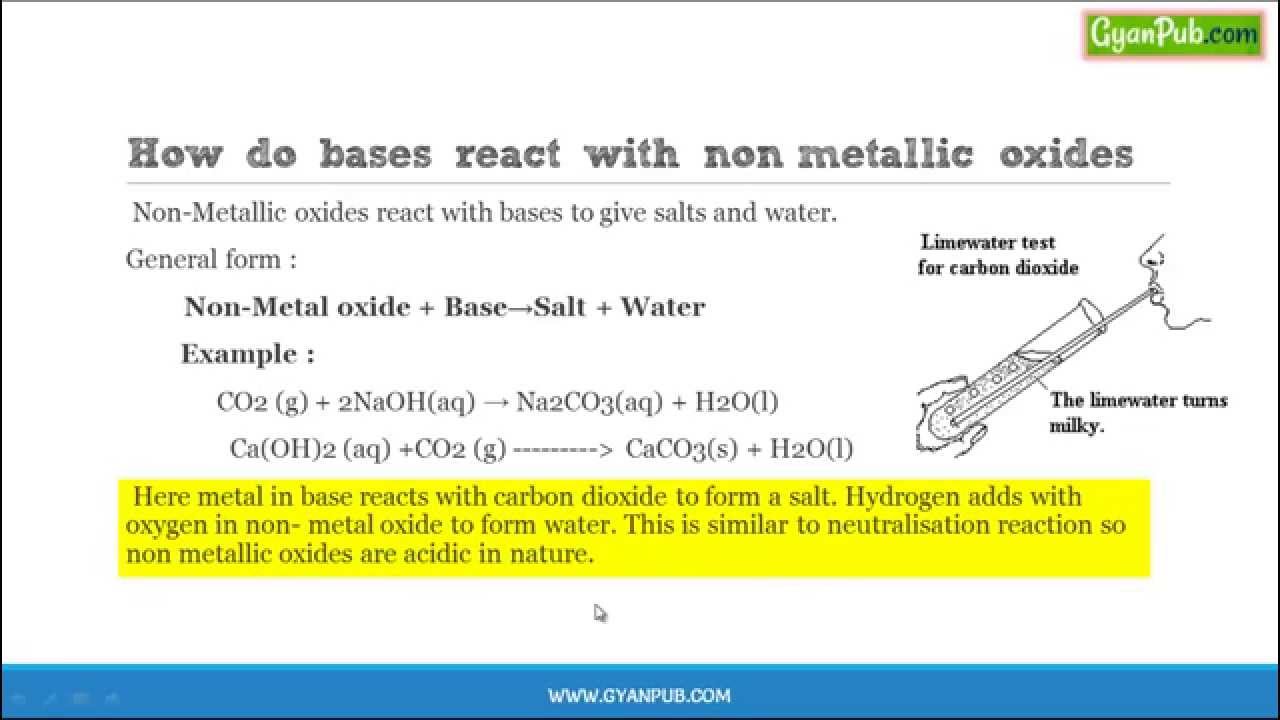
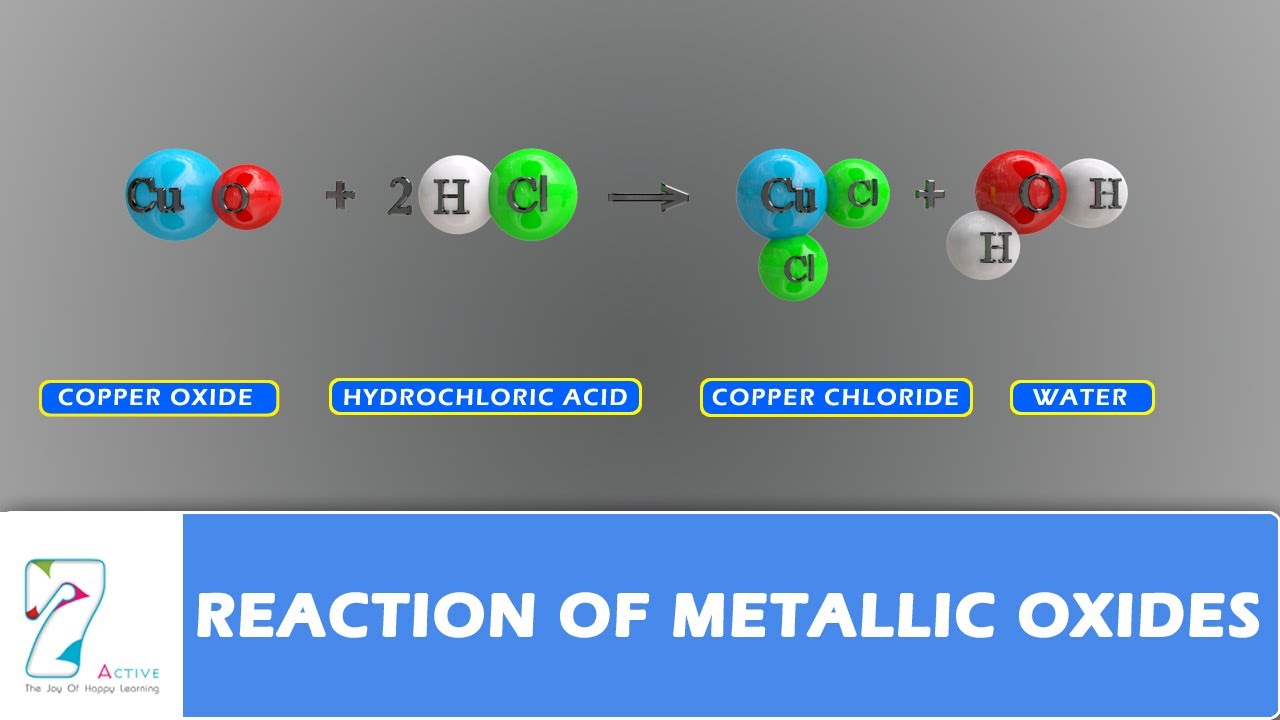
Metal Oxide And Acid Reaction Examples
Calcium is an alkaline earth metal.

Metal oxide and acid reaction examples. Since metals that are reactive form oxides that are stable some metal oxides must be electrolyzed to be reduced. This includes sodium oxide potassium oxide calcium. Covers many common types of chemical reaction with definitions and examples. Oxidation reduction acid base hydrolysis esterification and more.
The metal reactivity series metal reaction notes equations and explanations. The ideas behind the reactivity series of metals is introduced and what happens. Corrosion is a natural process which converts a refined metal to a more chemically stable form such as its oxide hydroxide or sulfide. It is the gradual.
Metal oxides are crystalline solids that contain a metal cation and an oxide anion. They typically react with water to form bases or with acids to form. Nitric acid is manufactured from ammonia and is a key chemical in the manufacture of fertilizers. Uses of nitric acid.
By far the principal use of nitric acid 80. Oxyacid any oxygen containing acid. Most covalent nonmetallic oxides react with water to form acidic oxides. That is they react with water to form oxyacids.